1 September 2025
Reading time [minutes]: 9
Technology and Applied Research
PCR and AI developments: new milestones for Ulisse Biomed
How the integration of PCR and artificial intelligence is revolutionising molecular diagnostics at Ulisse Biomed, from the bAPP™ AI-driven cloud to bCUBE™ portable devices, with impacts on quality, interoperability and clinical decision support.
Abstract
The convergence of PCR technology and artificial intelligence is opening up new frontiers in the Ulisse Biomed ecosystem. The company has evolved its bAPP™ platform into an AI-driven cloud hub integrated with the bCUBE™ portable device, enabling real-time analysis of Ct curves and advanced automation. In this article, we examine the achievements and future developments of this AI+PCR synergy: from assisted analysis of amplification curves (quality flags, drift identification, results confirmation support) to predictive quality, and the extension of these AI-driven solutions in decentralised contexts.
Snapshot
-
PCR (Polymerase Chain Reaction)
A gene amplification technique that allows DNA/RNA fragments to be exponentially copied, which is essential in molecular diagnostic tests.
-
Ct (Cycle Threshold)
Number of PCR cycles required for the amplification signal to exceed a defined threshold; indirect measure of the amount of target present in the sample.
-
bCUBE™
Portable device for real-time PCR developed by Ulisse Biomed, part of the Hyris System™ platform. Performs on-site molecular testing with integrated cloud connectivity.
-
bAPP™
Ulisse Biomed's cloud software platform for centralised management of diagnostic data. It provides KPI dashboards, APIs for integration (e.g. LIS/EMR) and AI modules for advanced analysis.
-
AutoML (Automated Machine Learning)
Machine learning approach in which algorithms and ML models self-configure and optimise, reducing human intervention. In diagnostics, AI models are adapted to new data on a continuous basis.
-
OEM (Original Equipment Manufacturer)
In the diagnostic context, it refers to third-party manufacturers with whom Ulisse Biomed can co-develop solutions (e.g., by integrating bCUBE/bAPP into other brands' systems) or provide white-label platforms.
-
LIS (Laboratory Information System)
Computerised laboratory workflow management system (patient records, sample tracking, reports). The integration of bAPP with hospital LIS ensures interoperability and automated data exchange.
- Introduction
- 1. Evolution of bAPP™: the cloud platform becomes AI-driven
- 2. AI and Ct curves: from automatic flags to assisted confirmation of results
- 3. AutoML and predictive maintenance: the lab learns by itself
- 4. AI in decentralised environments: from hospital to territory (and beyond)
- 5. Strategic implications: OEM co-development, regulatory validation and industrial partnerships
- Conclusion
Introduction
Every day, laboratories generate millions of data points: Ct amplification curves, machine logs, quality control flags, test metadata. Traditionally, this data remained confined to local information systems and its interpretation depended on the experience of biologists. Today, thanks to the integration of cloud and artificial intelligence, this data can be centralised, analysed algorithmically and transformed into clinical and operational value. Ulisse Biomed fully embraces this paradigm shift: with connected devices such as bCUBE and SaaS platforms such as bAPP, AI is not an accessory component but an integral part of the company's core business.
In Ulisse Biomed's Hyris System™ ecosystem, PCR becomes smart: raw amplification data is interpreted in real time by machine learning algorithms that assess its reliability, then integrate it into the clinical context via the cloud. This article explores the joint developments of PCR and AI within Ulisse Biomed, illustrating both the technological achievements already made and the new frontiers currently being implemented. We will begin by outlining the evolution of the bAPP platform in terms of AI-driven technology, before delving into how AI is applied to the analysis of Ct curves. We will then discuss the use of AutoML techniques for predictive device maintenance and continuous optimisation of diagnostic models, and the integration of Explainable AI (XAI) to ensure transparency in the process (essential for audit trails and reporting in regulated environments). [7]
Next, we will examine the use of these AI+PCR solutions in decentralised settings – from clinics to other decentralised contexts – highlighting how bringing artificial intelligence to the point-of-care can enable new services and improve response times. Finally, we will analyse the impact of this integrated AI+PCR pipeline on new standards of quality, interoperability and decision support, as well as the strategic implications for Ulisse Biomed and its stakeholders: opportunities for co-development with OEMs, regulatory validation considerations (IVDR, FDA) and potential industrial partnerships to accelerate adoption.
1. Evolution of bAPP™: the cloud platform becomes AI-driven
From the outset, Ulisse Biomed designed bAPP to be much more than traditional cloud-based PCR software. bAPP™ was created to be a centralised platform capable of orchestrating a network of distributed molecular devices (bCUBE) and transforming each test into actionable data. In recent years, bAPP has taken an evolutionary leap towards an AI-driven model: from classic dashboards and cloud repositories, it has moved on to the implementation of machine learning-based assisted analysis modules.
A significant milestone in this evolution was the introduction of the proprietary Intelligent Quality Control module. The module uses artificial intelligence algorithms to automatically control the quality of each PCR run: it verifies the shape of the amplification curve, checks threshold and baseline values, and cross-references these elements with historical and environmental parameters. If a test result is questionable or there are signs of possible errors (e.g., abnormal fluorescence), the system generates an alert flag and can discard or request a repeat test without human intervention. [4][5]
The benefits of this approach are beginning to be quantifiable: hundreds of bCUBE devices are now connected to the bAPP platform, forming a global network of real-time data. Every minute saved in confirming a PCR result can translate into more timely clinical interventions, faster isolation of an infectious patient, or immediate initiation of targeted therapy.
In summary, Ulisse Biomed's AI-driven bAPP platform embodies the fusion of IT and biotech: it collects diagnostic big data and converts it into smart data. Its evolution continues with a clear goal – to make every PCR test “not just a result, but an insight” – laying the foundations for generating added value both for the laboratory (quality control, operational optimisation) and for the healthcare system as a whole (epidemiological surveillance, new service models).
2. AI and Ct curves: from automatic flags to assisted confirmation of results
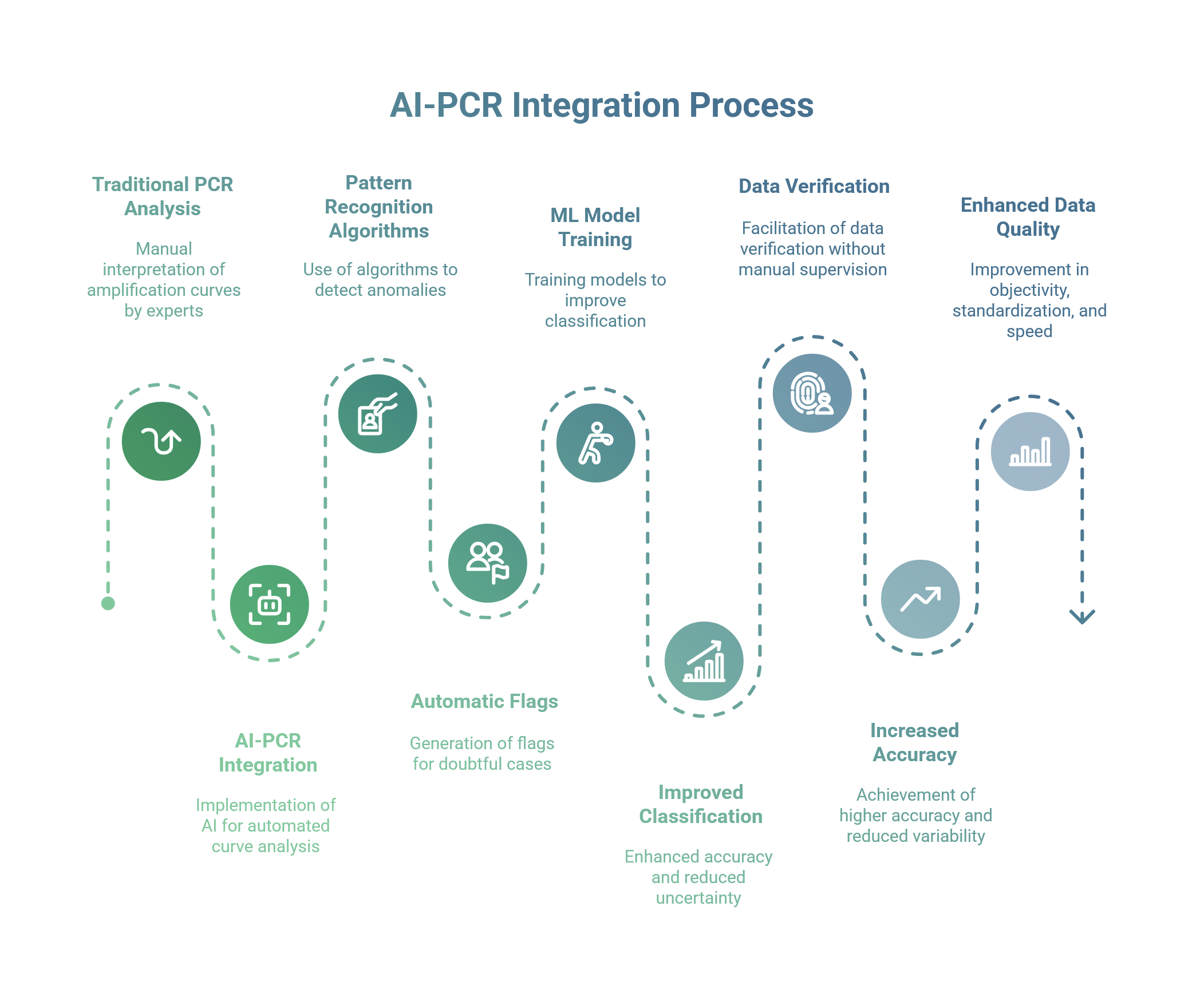
One of the areas where AI-PCR integration immediately shows its impact is in the automatic interpretation of amplification curves (Ct curves). In a traditional PCR test, the determination of a positive/negative result is based on exceeding a fluorescence threshold; but in many real-world cases, visual analysis of the curves by an expert is essential, for example to identify anomalies (atypical curves, background noise, suboptimal reactions) or to distinguish a true weak positive from an artefact. The shortage of experienced molecular biologists and the high workload during critical periods (such as pandemic peaks) make this process a bottleneck and a possible source of variability. Artificial intelligence can come to the rescue by standardising and automating the analysis of curves in real time [1].
Ulisse Biomed, through bAPP, has implemented pattern recognition algorithms trained on huge These algorithms generate automatic flags when they detect anomalous profiles. Ulisse Biomed's algorithms take into account multiple features (curve shape, second derivative, signal-to-noise ratio, etc.) and can classify the case as “doubtful”, suggesting further verification [2].
Recent studies confirm the effectiveness of ML approaches for interpreting qPCR curves. A study in Scientific Reports showed that appropriately trained ML models improve the classification of results and reduce “uncertain” outcomes compared to expert reading alone [1]. A further study in Molecules designed an AI system capable of detecting atypical profiles (contamination/artifacts), facilitating data verification without manual supervision for each reaction [2]. Applications on high-throughput panels (e.g., HPV) indicate that software automation + data science/AI increase accuracy and reduce variability in multi-well/multi-target readings [3].
Ultimately, the use of AI on Ct curves takes PCR data quality to a new level: objectivity, standardisation and speed [1][2][3]. For the scientific board and R&D, this means reliability (uniform criteria, audit trail); for laboratory staff, it means lightening the routine and focusing attention where it is needed.
3. AutoML and predictive maintenance: the lab learns by itself
In addition to analysing the results themselves, AI at Ulisse Biomed is also applied to the management of diagnostic infrastructure. A key element here is the introduction of predictive maintenance methodologies for devices. What exactly do we mean by this?
On the predictive maintenance front, AI analyses bCUBE machine logs and other parameters (operating temperatures, cycles performed, calibrations, etc.) to predict failures or performance drops before they occur. Each bCUBE device regularly sends information about its status to the cloud. By accumulating this data for hundreds of operating units in various contexts, bAPP has built a knowledge base of what is normal and what could be a precursor to a problem. For example, a slight but steady decline in recorded fluorescence could indicate that the optics need to be calibrated; an increase in heater calibration cycles could suggest that a Peltier element is about to fail. The AI correlates these patterns with maintenance histories: when it detects a combination of signals that has previously preceded a machine shutdown, it sends an alert to the support team or user laboratory, suggesting that preventive maintenance be performed on that specific bCUBE. This preventive intelligence can ensure much greater operational continuity. As studies on the subject have shown, machine learning models can analyse usage patterns and intervention history to predict when laboratory equipment is likely to fail, allowing for planned intervention and minimising downtime.
Ulisse Biomed is replicating this approach on its own scale: bCUBE devices are compact instruments, often located in remote sites or unattended by technicians (a decentralised laboratory may be located in a peripheral clinic or mobile facility). Having “AI agents” that constantly monitor the health of these devices is crucial to ensuring reliability in the field. Imagine a bCUBE installed in a hospital that performs molecular swabs during events or emergencies: it must operate 24 hours a day without surprises. If the predictive system on bAPP reports that that specific unit is showing signs of stress (perhaps due to intensive use, vibrations, etc.), a replacement or technical check can be scheduled for the next time the ambulance returns, before a failure occurs during a critical intervention. For a CTO or hospital innovation manager, predictive maintenance means reducing costs (fewer breakdowns = fewer emergency replacement devices, less downtime) and improving confidence in the new cloud model: a critical issue when adopting distributed diagnostic technologies is often the fear that the same level of technical support will not be available outside the central laboratory – AI helps to bridge this gap by acting as a “nervous system” that monitors every device in the field.
From a strategic point of view, the adoption of AI with predictive maintenance is part of Ulisse Biomed's broader vision: to offer not only a product (the diagnostic tool) but also a continuous and proactive service. This approach is in line with servitisation and SaaS (Software as a Service) models: the customer does not just buy a machine, but joins an intelligent ecosystem that ensures optimal performance throughout its life cycle. In terms of metrics, this can translate into more stringent SLAs (service level agreements) and recurring revenues for the company (think of advanced maintenance contracts based on the added value of AI). Not surprisingly, market reports indicate that AI-driven solutions in diagnostic services are enabling new pay-per-use business models and optimising operating costs by ~15%.
In conclusion, predictive maintenance is the flip side of AI applied “behind the scenes”: the former ensures that bAPP's analytical models remain up to date and accurate as the real world changes, while the latter ensures that the technical infrastructure (the bCUBE) remains functional and performs well. Both contribute to an essential goal: minimising human intervention in repetitive or predictable tasks, so that human resources can focus on innovation and critical decisions, while “the system takes care of itself” for everything else.
4. AI in decentralised environments: from hospital to territory (and beyond)
One of the historical challenges of molecular diagnostics is bringing testing closer to the patient, outside of centralised laboratories. Ulisse Biomed, with its portable bCUBE platform, has precisely this goal: to enable on-site testing in peripheral clinics, production sites and emergency contexts [8]. But performing PCR in decentralised contexts raises a question: how can the same quality and reliability be guaranteed as in a large centralised laboratory? The answer lies in the combination of a smart device and cloud AI.
In an emergency laboratory, there may not be a molecular biologist available to interpret the test. This is where Ulisse Biomed's AI comes in: the bCUBE performs the amplification cycle and sends the raw data in real time to bAPP, where algorithms analyse everything and provide support for reporting. In case of doubt, the system can also report: “result to be confirmed in a centralised laboratory”, if there is an inconsistency (this can happen, for example, if the AI module detects a possible problem with the sample or reagents). In any case, the user experience in the periphery remains simple and guided, while the complexity is managed behind the scenes by the AI.
Implementing AI in the field also means being able to centralise control even with distributed equipment: one of the lessons learned during the pandemic is that decentralised testing systems work better if there is centralised data supervision. Ulisse Biomed embodies this model: many small, widespread laboratories (the on-site bCUBE) but all connected to a central brain (bAPP cloud) that ensures uniformity in analysis criteria, simultaneous updates of AI protocols and comparison of performance between sites. A 2022 ECDC report emphasises that point-of-care testing requires centralised management to standardise and compare data between remote sites, ensuring consistent quality [6].
The introduction of AI in decentralised environments is therefore raising the bar for what can be done outside the laboratory: tests that previously required highly specialised personnel can now be performed at different points of care, with AI acting as an “invisible co-pilot”. From a clinical point of view, as also highlighted by recent pilot experiences, this translates into a tangible improvement in clinical timeliness and even a reduction in avoidable hospitalisations. For example, decision support systems integrated into medical records, which aggregate real-time decentralised laboratory data with vital signs and medical history, can generate predictive alerts (e.g., risk of sepsis) before the situation deteriorates. A molecular test performed “on the spot” and analysed with AI can anticipate life-saving treatment by hours.
In strategic terms, Ulisse Biomed's positioning in this AI+POC (AI + Point of Care) segment addresses several drivers: the need for healthcare systems to dehospitalise where possible, bringing specialist skills to the local area; the need to save resources (moving data instead of patients or samples); and the regulatory and political push towards community-based medicine. It also enables new business models: for example, one could imagine a network of private clinics adopting the Ulisse Biomed solution to offer advanced tests with added value (immediate reporting + predictive advice), or partnerships with telemedicine and home care companies, where bCUBE+AI becomes part of a telehealth kit. In the OEM sector, Ulisse Biomed could supply its AI+PCR technology to manufacturers of mobile medical equipment (such as high-tech ambulance manufacturers or suppliers of systems for the armed forces and civil protection) by integrating it into their vehicles and facilities. The possibilities are vast and all converge on one point: AI makes decentralised diagnostics truly practical and reliable, bridging the gap that has often limited its large-scale adoption until now.
5. Strategic implications: OEM co-development, regulatory validation and industrial partnerships
The joint PCR+AI innovation at Ulisse Biomed has not only technological implications, but also important strategic implications for the company's business and partnership ecosystem. Let's examine three key areas: opportunities for co-development with OEMs, regulatory validation challenges, and possible industrial partnerships enabled by this platform.
Co-development with OEMs: Ulisse Biomed positions itself not only as a manufacturer of its own solutions, but also as a technology partner for other players (OEMs) who want to integrate components of the platform (hardware or software) into their offerings. The AI-driven evolution of the system increases the attractiveness of such collaborations. A manufacturer of traditional diagnostic tools, for example, could see value in adopting the bAPP cloud platform to give its devices a “smart” look: instead of developing a cloud infrastructure with AI from scratch, it could co-develop with Ulisse Biomed, bringing its own reagents or protocols to bCUBE and leveraging bAPP's AI for analysis and digital services. This integrated OEM model creates win-win synergies: Ulisse Biomed expands the reach of its platform (and related SaaS revenues) without having to cover every segment or specialised application on its own; the OEM partner enriches its product with advanced features (cloud, AI, interoperability) quickly and at a lower cost than developing them in-house. Ulisse Biomed already offers white-label and OEM development services, and the addition of AI can be a powerful selling point. Let's imagine a veterinary diagnostics company that wants a portable system for animal diseases: by adopting the Ulisse Biomed ecosystem, it would immediately have the ability to offer veterinarians AI analysis of curves, predictive alerts on animal epidemics, etc., strongly differentiating itself in the market. Three differentiating factors that Ulisse Biomed can offer OEMs are:
- integrated end-to-end proprietary technology (device + cloud + AI, no other competitor provides everything in one stack);
- competitive costs per test due to automation (already today, Ulysses systems have costs/tests 400-500% lower than traditional large brand solutions;
- Modularity and scalability (a partner can start with a few devices and grow, add test panels, etc., relying on a scalable cloud infrastructure).
In summary, Ulisse Biomed's OEM value proposition becomes extremely attractive: ‘we bring laboratory accuracy everywhere, with a ready-made, intelligent platform’. In fact, Ulisse Biomed positions itself as an enabler for others – a strategy that could exponentially expand its market presence (each OEM partner opens up new channels and geographical markets). Industrial partnerships: Finally, the partnership aspect. Ulisse Biomed's new achievements in PCR+AI are attracting the interest not only of end customers but also of complementary big players. For example, cloud computing and healthcare IT companies may want to integrate Ulisse solutions into their ecosystems: a partnership with a global cloud provider could ensure scalability and territorial compliance (think of the sovereign cloud requirements in certain countries – collaborating with local operators to host bAPP on national clouds may be key to entering those markets). Or collaborations with pharmaceutical companies: in clinical trials, having portable PCR with AI means collecting data faster and more uniformly – Ulisse Biomed could enter into agreements to provide its technology in multicentre studies (the company's Development Services & OEM division already offers tailor-made services, and AI enhances this offering with immediate analysis of experimental data). The finance/insurance world could also be interested: imagine health insurance companies providing their customers with home diagnostic devices (perhaps a bCUBE “home edition”) for prevention – this would require an AI infrastructure to monitor and alert, and Ulisse Biomed would have the know-how to implement it in partnership.
Last but not least, there is the standardisation aspect: defining new quality and interoperability standards gives Ulisse Biomed a say in industry forums (e.g. consortia for diagnostic interoperability, collaborations with organisations such as the WHO or CDC on pilot projects). For example, Ulisse Biomed could provide the WHO with AI+PCR platforms for active epidemiological surveillance programmes in emerging countries, contributing to new rapid response protocols (combining decentralised testing and AI for real-time tracking). Such partnerships increase international visibility and also facilitate access to foreign markets with the support of government agencies.
Conclusion
For Ulisse Biomed, the integration of PCR and AI represents more than just a technological advance: it is a true paradigm shift in molecular diagnostics. The use cases and achievements described – from automated quality control that reduces reporting times, to predictive maintenance that ensures continuous operation, to deployment in ambulances with real-time decision support – demonstrate how the “cloud-first, AI-driven” vision is becoming a reality in the Ulisse ecosystem [7].
This evolution brings measurable benefits: reduced response times (TAT reduced by up to 30% in real settings), reduction in errors and inconsistencies (AI eliminates human variability and flags anomalies before they become problems), improved compliance (digital audit trails, integrated traceability, IVDR/FDA compliance by design) and the activation of new service models (diagnostic SaaS, pay-per-test, regional tele-diagnostics).
Important challenges remain, of course: from cybersecurity (it is essential to maintain encryption and certifications, given that data travels on the cloud) to change management (persuading laboratories and clinicians to trust AI – to be addressed with targeted training). But the direction has been set: molecular diagnostics in the near future will be connected, intelligent and decentralised. Ulisse Biomed, with its Hyris System™ platform, is at the forefront of this movement, integrating hardware, consumables and software into a single high-value data stream.
Ultimately, the joint developments of PCR and AI are no longer a future option, but a necessary evolution that is already underway. Laboratories and healthcare stakeholders who embrace this evolution will be able to offer faster, more accurate and contextualised diagnoses, creating a competitive advantage and, more importantly, improving patient outcomes. Ulisse Biomed is demonstrating in the field that every molecular data point can become a clinical and strategic insight: we are moving from the binary values of a test to predictive knowledge that is useful to the entire healthcare ecosystem. The “new milestones” achieved are therefore only the beginning of a journey in which AI and PCR together will redefine the standards of how we test, interpret and use genomic information for health.
Sources and Bibliography
[2] Villarreal-González R., Acosta-Hoyos A.J., Garzon-Ochoa J.A., et al. Anomaly Identification during PCR for Detecting SARS-CoV-2 Using Artificial Intelligence Trained from Simulated Data. Molecules. 2021;26(1):20. doi: 10.3390/molecules26010020 — MDPI
[3] Pereira A.R., Redzic N., Van Vooren S., et al. Development, Validation, and Implementation of an Augmented Multiwell, Multitarget Quantitative PCR for HPV Genotyping through Software Automation, Data Science, and AI. J Mol Diagn. 2024;26(9):781-791. doi: 10.1016/j.jmoldx.2024.05.012 — PubMed
[4] Lorde N., Kalaria T. Machine Learning for Patient-Based Real-Time Quality Control (PBRTQC), Analytical and Preanalytical Error Detection in Clinical Laboratory. Diagnostics. 2024;14(16):1808. doi: 10.3390/diagnostics14161808 — MDPI
[5] Duan X., Zhang M., Liu Y., et al. Next-Generation Patient-Based Real-Time Quality Control Models. Ann Lab Med. 2024;44(5):385-391. doi: 10.3343/alm.2024.0053 — annlabmed.org · PMC
[6] European Centre for Disease Prevention and Control (ECDC). Assessment of point-of-care testing devices for infectious disease surveillance, prevention and control – a mapping exercise. 27 Apr 2022. (Report). Link — ECDC
[7] Spies N.C., Farnsworth C.W., Wheeler S., McCudden C.R. Validating, Implementing, and Monitoring Machine Learning Solutions in the Clinical Laboratory Safely and Effectively. Clinical Chemistry. 2024;70(11):1334-1343. doi: 10.1093/clinchem/hvae126 — Oxford Academic · ARUP Laboratories
[8] Yang S., Wen W. Lyophilized Ready-to-Use Mix for the Real-Time Polymerase Chain Reaction Diagnosis. ACS Appl Bio Mater. 2021;4(5):4354-4360. doi: 10.1021/acsabm.1c00131 — PubMed