10 August 2025
Reading time [minutes]: 12
Microbiota between Diagnostics and Nutraceuticals
Gut microbiota test: PCR innovations
Portable qPCR, < 3 h turnaround and bAPP™ cloud connect anti ageing clinics and OEM nutraceuticals in an AI driven ecosystem
Abstract
The gut microbiota is at the centre of new diagnostic and nutraceutical approaches. Ulisse Biomed has developed a rapid molecular test based on quantitative PCR (qPCR) that innovates gut microbiota diagnosis by making it decentralised, modular and fast. The Hyris System™ - comprising the bCUBE™ portable mini-laboratory, the bAPP™ cloud portal and ambient-stable reagents - enables highly specific quantification of key gut bacteria (genus Lactobacillus, Akkermansia muciniphila, Faecalibacterium prausnitzii, Bifidobacterium) with a reporting time (TAT) reduced to a few hours. Thanks to this integrated platform, anti-aging clinics, specialist laboratories and OEM partners in the nutraceutical sector can perform high-sensitivity on-site tests without the need for centralised laboratories. The article analyses how this innovation improves nutritional coaching programmes and customised diet plans, enables B2B2C models (co-branded kits and Diagnostics-as-a-Service) and supports prevention and anti-inflammatory check-ups. An interview with Gabriele Salaris, Head of Marketing & Sales at Ulisse Biomed, explores the strategic vision and positioning of this solution in the global personalised health market.
Introduction
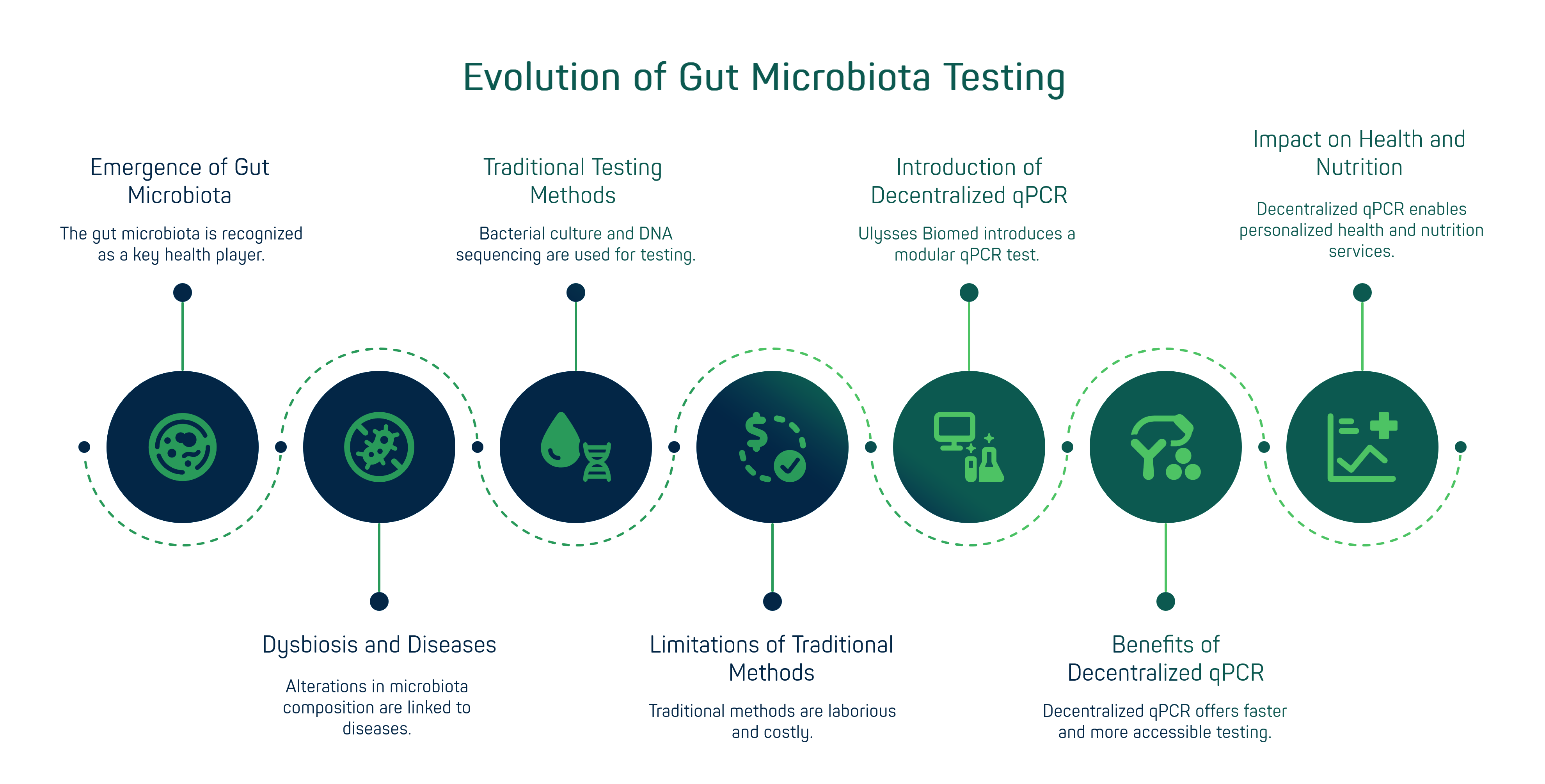
In recent years, the gut microbiota has emerged as a key player in human health, influencing metabolism, immunity and even systemic inflammation[3][4]. Alterations in the composition of the microbiota (dysbiosis) are associated with numerous diseases: for example, chronically low levels of Faecalibacterium prausnitzii - a butyrate-producing bacterium with anti-inflammatory properties - have been found in inflammatory bowel conditions such as Crohn's disease and ulcerative colitis[5]. Likewise, the presence of Akkermansia muciniphila correlates positively with improved intestinal barrier integrity and a healthier metabolic profile [4].
In parallel, bacterial genera traditionally known as beneficial - Lactobacillus and Bifidobacterium - have always populated commercial probiotics and exerted positive effects on intestinal homeostasis [6]. In summary, species such as Lactobacillus, Bifidobacteria, Faecalibacterium and Akkermansia are now recognised as important biomarkers of a state of intestinal balance or imbalance. This new awareness has generated a strong demand for microbiota testing in both clinical and consumer settings. However, traditional methods have several limitations. Bacterial culture, although the historical mainstay of clinical microbiology, is laborious and often fails to grow anaerobic or low abundance species typical of the microbiota [7][8]. On the other hand, analyses based on DNA sequencing (e.g. 16S rRNA amplicon or metagenomics) require the sample to be sent to specialised laboratories, with reporting times in the order of weeks, high costs and complexities of data interpretation. Commercially available direct-to-consumer kits, often based on sequencing, also suffer from a lack of standardisation and clinical validation: an international consensus pointed out that many direct-to-consumer microbiome tests have no proven clinical value or clear regulation [11]. The risk is to waste patients' and professionals' resources, generating confusion without real benefit.
Against this backdrop, Ulysses Biomed - an integrated biotech group focused on decentralised molecular diagnostics - has introduced a major innovation: a modular qPCR test for evaluating the gut microbiota. Based on the Hyris System™ technology ecosystem, this approach aims to democratise microbiota diagnostics, bringing it out of large laboratories: anti-aging clinics, nutrition clinics and even into the hands of nutraceutical partners who want to offer customised services to their clients. In the following paragraphs, we will examine the technical aspects of this solution - from analytical sensitivity and specificity to the hardware/software components that make it possible - and the strategic implications for the nutraceutical and personalised prevention sector. In particular, we will evaluate how decentralised qPCR tests can bridge the gap between diagnostics and nutrition, enabling new business models and improving the quality of gut wellness pathways.
1. Technical development: the decentralised qPCR platform
Ulisse Biomed's Hyris System™ is an integrated diagnostic ecosystem consisting of hardware, cloud software and innovative reagents, designed to deliver rapid and accurate molecular analysis in any setting [12][13]. At the heart of the system is the bCUBE™ portable device, a real-time PCR thermal mini-cycle measuring approximately 10x10x12 cm. This high-performance portable laboratory supports up to 36 simultaneous reactions and is equipped with multi-target optical channels, enabling multiplex analysis without sacrificing sensitivity [14][15]. The bCUBE requires no calibration or complex installation: it connects via Wi-Fi/Ethernet and is immediately operational, enabling plug-and-play diagnostics even in small laboratories or medical practices.
In parallel, the bAPP™ cloud portal serves as both user interface and data analysis platform. Through bAPP, the operator starts the test, follows the step-by-step instructions and obtains an automatically interpreted report. Built-in artificial intelligence algorithms analyse the amplification curves in real time and provide a clear result ('Faecalibacterium amount below normal', 'A. muciniphila in optimal ranges', etc.), reducing the dependency on specialised personnel [16]. All data are synchronised on the cloud in real time, meaning that a nutritionist or doctor can view the results immediately, even remotely, immediately after the test is completed [17]. In terms of cybersecurity and privacy, the platform complies with medical standards, ensuring that even in decentralised scenarios sensitive data is protected.
A distinctive technical element concerns reagents and the sample preparation workflow. Ulisse Biomed uses cartridges pre-loaded with proprietary lyophilised reagents that are stable at room temperature [18]. This eliminates the need for the cold chain for transport and storage, traditionally a major obstacle to the spread of molecular testing outside large centres [18]. In practice, amplification kits and primers specific to the target bacteria in the microbiota (e.g. marker sequences of Lactobacillus spp., A. muciniphila, F. prausnitzii, Bifidobacterium spp.) are ready-to-use and shelf-stable, allowing clinics and partner companies to run tests without complex laboratory infrastructure.
In terms of analytical performance, the test offers sensitivity and specificity comparable to laboratory reference methods. Real-time qPCR technology, especially if well designed, achieves very high levels of sensitivity: recent studies have shown that targeted qPCR assays can detect bacterial targets in faecal DNA with detection limits in the order of 10^3-10^4 cells per gram[1], exceeding the detection threshold of standard metagenomic methods (which are less sensitive in terms of absolute abundance)[2]. Thanks to highly specific primers and specially designed molecular probes, the test accurately distinguishes microbial groups of interest without unwanted cross-reactions. For example, Faecalibacterium detection is calibrated to unique sequences of F. prausnitzii, ensuring that signals from other related butyrogenic species present in the gut are not captured. The specificity of each primer/probe set is validated in silico and in vitro on pure strains, ensuring that only target species produce a positive signal. The overall accuracy of the Hyris system has been verified in clinical settings: validation studies have compared the results of the bCUBE with those of standard laboratory thermal cyclers and found an almost superimposable analytical concordance[20].
One clear strength of the Ulisse Biomed test is its speed. The entire turnaround time (TAT), i.e. the time from sampling to result, is less than 3 hours. Specifically, the PCR amplification phase takes approximately 90-120 minutes thanks to optimised thermal cycles (the bCUBE uses rapid thermal controls and a reduced reaction volume to speed up the protocol). The remaining time covers the minimal initial sample preparation and data analysis/transmission once the PCR is complete. In less than three hours, therefore, the clinician or nutrition consultant has a quantitative report of the main indicators of the gut microbiota. This is a dramatic improvement over traditional microbiome tests, which take days or even weeks. For example, an NGS test sent to an external laboratory typically requires sample shipment, DNA extraction, mass sequencing, bioinformatic analysis and reporting: a process that, even in the best cases, takes several days. With the decentralised qPCR solution, however, the information travels immediately to the cloud (not the sample), enabling a just-in-time approach to diagnostics.
Thanks to these technical features - portability, speed, simplicity and stability - the Ulisse Biomed microbiota test lends itself to broad application scenarios previously impractical with traditional technologies. Below we explore how this innovation can be exploited both in clinical and prevention contexts (e.g. anti-aging programmes, targeted check-ups) and through OEM partnerships in the nutraceutical sector to create new service models.
2. Clinical and nutraceutical impact: towards the personalisation of gut health
The introduction of rapid and decentralised microbiota testing opens up exciting perspectives in the fields of preventive medicine, wellness and personalised nutrition. An emblematic use case is that of anti-ageing and preventive clinics. In these centres, patients engaged in longevity and wellness programmes wish to comprehensively monitor their health status, including the inflammation- and metabolism-related component of the gut microbiota.
With Ulisse Biomed's qPCR test, an anti-aging clinic can also integrate real-time microbiotic assessment into the patient's check-up. For example, during the initial visit, the doctor takes a small stool sample (e.g., via a stool swab collected by the patient on the same day) and analyses it immediately on-site with bCUBE. In less than 3 hours, while the patient completes other tests, the clinic obtains a quantitative profile of their key beneficial gut bacteria. If the results show, for example, a very low level of Faecalibacterium prausnitzii – often associated with pro-inflammatory states [5] – the nutritionist can proactively intervene, recommending a diet rich in soluble fibre and polyphenols (to feed butyrate producers) or targeted supplements such as specific prebiotics. Similarly, a deficiency in Akkermansia, a bacterium associated with weight control and metabolic health, could lead to the adoption of certain nutraceuticals (e.g. polyphenolic extracts such as blueberry, which promote Akkermansia) or future new-generation probiotics containing A. muciniphila itself, currently under investigation. In practice, the test provides actionable indicators during the clinical appointment, allowing for an unprecedented level of personalisation of the nutritional plan. The nutrition coach or dietitian can precisely calibrate the diet and any supplements based on the individual's “microbiota dashboard” and then monitor progress by repeating the test a few months later. This dynamic monitoring was not possible with traditional tests, which were too slow and expensive to be repeated frequently. Now, however, it is conceivable to include microbiota measurement as a periodic KPI in nutritional coaching programmes (e.g., at the beginning and end of a 3-month programme), measuring the impact of diet and supplements on the rebalancing of the gut flora.
Another area of application is that of targeted prevention and functional medicine pathways. Many centres of preventive gastroenterology or integrative medicine are looking for early markers of inflammatory and metabolic risk. Classical tests such as PCR (C-reactive protein) or lipid panel provide systemic information, but the gut microbiota offers a unique window into the patient's state of eubiosis/disbiosis. Incorporating the 'microbiota profile' into annual screenings could help identify individuals with initial imbalance (e.g. reduced microbial diversity or lack of anti-inflammatory bacteria) even before clinical symptoms occur. Consider patients with a family history of chronic inflammatory bowel disease: an anti-inflammatory check-up could include, alongside faecal calprotectin, also the quantification of F. prausnitzii, given its known decrease in inflammatory flares[5]. In the case of values below a certain threshold, the prevention centre could activate in-depth nutritional counselling or probiotic therapies in an attempt to restore the balance and avert the evolution towards pathology. Similarly, in metabolic prevention pathways (e.g. for pre-diabetic or overweight subjects), monitoring Akkermansia and Bifidobacterium provides clues as to how to modulate diet and lifestyle: increase prebiotic fibres if Bifidobacterium is low, or evaluate the effectiveness of a nutraceutical intervention by measuring the relative increase in these beneficial populations after the intervention.
3. OEM integration and new B2B2C models
In addition to direct use in clinical settings, Ulisse Biomed's innovation offers opportunities for strategic partnerships in the nutraceutical and personal health sectors. The OEM (Original Equipment Manufacturer) model allows third-party players to integrate the Hyris system into their own services or products on a co-branded basis. Let's imagine a food supplement company specialising in premium probiotics: thanks to Ulisse Biomed, it could develop a microbiota kit under its own brand (powered by Hyris System™) to offer to customers together with its probiotic cycle. The end customer would then be able to perform an initial test at home or at an affiliated collection point, obtain a cloud-based profile of their 4-5 crucial gut bacteria, receive a personalised report explaining the results and, based on these, follow a tailor-made supplementation programme. A second follow-up test could then verify the effectiveness of the probiotic in colonising the gut (e.g., an increase in Lactobacilli and Bifidobacteria compared to baseline). This approach represents an innovative B2B2C model: the B2C nutraceutical company enriches its offering with an advanced diagnostic service, differentiating itself in the market, while Ulisse Biomed provides the technology platform and reagents on an OEM basis. In essence, diagnostics becomes an integral part of the nutraceutical product, creating a more comprehensive, data-driven user experience.
From an operational standpoint, Ulisse Biomed supports OEM partners in a variety of flexible ways. Some choose to purchase bCUBE devices and reagent kits for use in their own affiliated centres (the classic model for selling instruments and consumables). Others opt for as-a-Service formulas: for example, a monthly subscription that includes access to the cloud platform and technical support, without having to invest in hardware upfront. This “Diagnostics-as-a-Service (DaaS)” option is attractive to companies that want to launch a pilot microbiota testing service with low initial costs and then scale up according to demand. Ulisse Biomed has designed its ecosystem to facilitate this integration: the modularity and scalability of the Hyris system allow you to start small (e.g., a single device in a nutrition clinic) and then grow to a network of dozens of test points, all managed on the same cloud infrastructure. The bAPP can be configured to suit the partner's user experience: the interface and reports can be customised with the OEM brand, different languages can be added, and data can even be linked to the partner's dashboards or apps via APIs and integrations (note: Ulisse also offers a bGATE™ component to integrate third-party tools or external data streams into its cloud[23][24]). This technological openness means that a partner can, for example, combine the results of the microbiota test with other data from a wellness app (diet, physical activity, biometric parameters) to provide even more personalised recommendations to its customers.
Another competitive advantage of the model offered is the speed of innovation. Instead of having to develop a diagnostic platform from scratch in-house – with significant investments in R&D, validation and regulatory processes – partners can rely on a solution that is already certified and on the market, allowing them to focus on their core business. For example, a chain of specialised laboratories could quickly activate a same-day microbiota analysis service using bCUBE and Ulisse kits, tapping into growing demand from patients who are knowledgeable about the microbiome. Alternatively, telemedicine companies could offer their users a home kit with integrated video consultation with a doctor to discuss the results after 90 minutes. The possibilities are endless and all converge on one point: decentralisation and diagnostic-nutraceutical convergence are creating new healthcare ecosystems in which data, hardware and services merge. Ulisse Biomed, positioning itself as a technology enabler with its Hyris System™, is at the centre of this convergence.

Gabriele Salaris
Head of Marketing & Sales di Ulisse Biomed
4. Interview
Q: What is the main innovation that Ulisse Biomed introduces in gut microbiota diagnostics compared to traditional methods?
A: In the past, microbiota assessment required complex tools and long timeframes, often only accessible in large centralised laboratories. With our portable qPCR platform, we are reversing this paradigm. The key innovation is that we have made microbiota testing possible anywhere and in less than three hours, without sacrificing data quality. We have integrated hardware, reagents and the cloud into a single, coherent ecosystem, so that clinics of any size, or even nutraceutical companies, can offer advanced genetic analysis services without having to build a laboratory from scratch. The result is a democratisation of diagnostics: we bring precision testing directly “into the field” – whether it's a nutritionist's office or a partner company's headquarters – while maintaining high analytical standards. Compared to traditional methods, we avoid the end customer having to send samples and wait weeks, and we give professionals an immediate decision-making tool. Furthermore, the specificity of our tests eliminates the “black box effect” of many broad microbiome tests: the doctor obtains clear measurements of well-defined bacteria with known clinical significance, which can be immediately translated into practical advice. In short, innovation is not only technological but also workflow-based: speed, simplicity and actionability of results, all in one package.
Q: Your target audience ranges from preventive clinics to OEM nutraceutical partners. How do you manage to meet such diverse needs with a single platform?
A: The secret was to design the platform from the outset with a multi-sector, modular approach. The DNA of the technology is the same – nucleic acid analysis – whether you are looking for an intestinal bacterium or checking the purity of a medicinal plant. What changes is how we package the experience around the technological core. For clinics, we focus on compliance and ease of use: bCUBE and bAPP comply with medical standards and reporting is designed to integrate into the clinical pathway (think, for example, of the possibility of automatically entering results into digital health records). For nutraceutical and OEM partners, we offer integration flexibility: from co-branding to the option of choosing specific test panels (an OEM may want to add other microbial or genetic targets relevant to its product) to tailor-made commercial models. Some prefer to purchase the device, others opt for an all-inclusive subscription model – and we support both options. Furthermore, thanks to the cloud, scalability is built in: a partner can start with a single bCUBE unit at a pilot site and then expand the network to dozens of devices in different countries, all while continuing to use the same centralised cloud platform for data. This means, for example, that a company can collect all the tests carried out at various locations in a single aggregated database – a goldmine of data for epidemiological or product effectiveness analysis (anonymised and in compliance with privacy regulations, of course). So, in a nutshell, we serve different markets because we have built a versatile ecosystem: portable hardware, configurable software and customisable services, while maintaining a common core that guarantees reliability and consistency. It's like an operating system that can run different applications depending on the user: our “app” for nutritionists highlights certain aspects, while the one for nutraceutical companies may emphasise others, but the underlying platform is the same.
Q: From a strategic marketing perspective, how do you position this solution in the competitive landscape? What is your Unique Selling Proposition (USP) for investors and partners?
A: Our USP lies in the combination of two elements that are rarely found together: integrated verticality and agility. Many competitors are strong in a single aspect – some have the most powerful sequencer, others have the most extensive test menu, and others have the most user-friendly software. Ulisse Biomed, thanks to the combination of Hyris and Ulisse expertise, offers everything together: hardware + software + reagents + cloud, developed in-house and perfectly orchestrated[26][27]. This creates a significant barrier to entry: replicating a complete ecosystem requires know-how in several disciplines and years of development. At the same time, we are a lean and innovative company: in just over a year since the Hyris-UBM integration, we have launched new products (such as STI panels for sexually transmitted infections) and signed partnerships in emerging sectors such as medical cannabis and personalised medicine[28][29]. This demonstrates to investors that we are not a static idea but a living platform capable of adapting and penetrating various growing markets. In the specific field of the microbiota, we are positioning ourselves as a first mover in making quality distributed diagnostics truly viable. There are large companies focused on consumer sequencing and start-ups focused on individual aspects of the microbiome, but few offer an end-to-end solution like ours: from the certified portable device to the cold chain-free reagent to AI for automatic interpretation. For partners and investors, the message is that Ulisse Biomed is building the future of decentralised diagnostics – not just in the microbiota – a future where rapid, connected and user-friendly tests will be the norm. We are at the intersection of biotech and digital health, combining the best of both worlds. This hybrid positioning, together with our proven execution capabilities, makes us a unique opportunity for those who believe in the convergence of diagnostics and personalised healthcare.
Conclusions
The evolution of gut microbiota diagnostics proposed by Ulisse Biomed embodies a paradigm shift in how we think about preventive care and personalisation in healthcare. By breaking down logistical and time barriers, the rapid, decentralised qPCR test transforms the microbiota from a difficult-to-access parameter into a real-time indicator that can be used by both doctors and informed consumers. This means that valuable data on gut health – once confined to laboratory reports received weeks later – can now guide immediate decisions: from adjusting a diet to choosing a specific supplement to the need for further clinical investigation as a preventive measure. All this while maintaining the accuracy standards of advanced molecular diagnostics. In an era in which precision medicine and the “one-health” model (which integrates human health, nutrition and the environment) are gaining prominence, solutions such as Ulisse Biomed's serve as a bridge between the laboratory and everyday life.
The ability to bring sophisticated genetic analysis to the community, connected through the cloud, creates a network of widespread diagnostic sentinels that feed into a collective knowledge base. Such a connected ecosystem can not only improve individual wellness journeys (e.g., by providing rapid feedback in a personalised nutrition programme), but also generate anonymous big data that is useful for public health and research. Imagine a future where dozens of distributed microbiota testing points share trends in real time: we could identify correlations between lifestyles and microbiotic profiles at the population level, or monitor the effect of new functional foods on a large scale. From a strategic point of view, Ulisse Biomed is demonstrating how the vertical integration of different technologies – miniaturised hardware, innovative reagents, artificial intelligence and cloud platforms – can give rise to highly innovative and disruptive products. This integrated approach allows a delicate balance to be maintained: decentralisation without compromising on quality. Instead of pitting centralised models against point-of-care models, the Hyris solution combines them synergistically, offering “near-patient” testing with the rigour and connectivity of a large laboratory[30][31]. The gut microbiota, at the crossroads between diagnostics and nutraceuticals, is the ideal use case to demonstrate this value.
In conclusion, ‘Gut microbiota test: PCR innovations’ is not just the title of a new service, but represents a piece of a larger picture: that of personalised, proactive and data-driven healthcare. A picture in which different players – clinics, nutraceutical companies, laboratories and patients themselves – collaborate through common platforms to improve health outcomes. With its vision and technology, Ulisse Biomed is at the forefront of this movement, offering concrete tools to translate knowledge about the microbiota into tangible actions for prevention and well-being. The result is a diagnostic model that is “ultra-connected” to the future of health, where knowing your microbiota becomes as simple as taking a blood test, and where every piece of data generates immediate value for the individual. The challenge and opportunity will be to extend these principles to other diagnostic areas, continuing to innovate without losing sight of the ultimate goal: to make healthcare more accessible, timely and tailored to each of us.
Sources and Bibliography
- Martín R. et al. (2023). Faecalibacterium: a bacterial genus with promising human health applications. FEMS Microbiol Rev, 47(4): fuad039. Low levels of Faecalibacterium correlate with inflammatory conditions (IBD, etc.)[5].
- The Gut Microbiotassay study – Hansen et al. (2013). BMC Genomics 14:788. qPCR is noted for high specificity, sensitivity, broad dynamic range, and quick performance at relatively low cost[32].
- Porcari S. et al. (2025). International consensus statement on microbiome testing in clinical practice. Lancet Gastroenterol Hepatol 10(2):154–167. Many DTC microbiome tests lack regulation or proven clinical value, risking waste of resources[11].
- Lee JY et al. (2022). Sci Reports 12:7838. prausnitzii ~5% of healthy gut microbiota (but lower in IBD, CRC) and A. muciniphila ~3% in healthy gut, produces SCFAs, modulating metabolism and immunity[3][4]. Lactobacillus and Bifidobacterium are traditional probiotics widely studied[6]; emerging next-gen probiotics like Akkermansia and Faecalibacterium show new therapeutic potential[33].
- Li F. et al. (2024). Microbiome 12:168. qPCR vs ddPCR for gut bacteria: LOD ~10^4 cells/g (qPCR) with standard extraction, improved to ~10^3 cells/g with optimized protocol; qPCR showed broader dynamic range and much lower LOD than 16S/NGS methods[1][2].
- Ulisse Biomed – Hyris System™ Ecosystem (2025, Insights article). Proprietary lyophilised reagents are stable at room temperature, removing need for cold chain[18]. bAPP cloud portal provides step-by-step guidance and automatic result interpretation, reducing need for specialized staff[16]. Data from distributed bCUBE devices sync in real time to the cloud, enabling immediate remote access to results[17].
[1] [2] Highly accurate and sensitive absolute quantification of bacterial strains in human fecal samples | Microbiome | Full Text
https://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-024-01881-2
[3] [4] [6] [33] Oral administration of Faecalibacterium prausnitzii and Akkermansia muciniphila strains from humans improves atopic dermatitis symptoms in DNCB induced NC/Nga mice | Scientific Reports
[5] Faecalibacterium: a bacterial genus with promising human health applications | Scinito
https://app.scinito.ai/article/W4384276655
[7] [8] [9] [10] Current Capabilities of Gut Microbiome–Based Diagnostics and the Promise of Clinical Application - PMC
https://pmc.ncbi.nlm.nih.gov/articles/PMC8206793/
[11] International consensus statement on microbiome testing in clinical practice - PubMed
https://pubmed.ncbi.nlm.nih.gov/39647502/
[12] [13] [14] [15] [16] [17] [18] [19] [20] [21] [22] [23] [24] [25] [26] [27] [28] [29] [30] [31] ID15 - The Ulisse Biomed Ecosystem: the Hyris System™ modular diagnostic platform
[32] The Gut Microbiotassay: a high-throughput qPCR approach combinable with next generation sequencing to study gut microbial diversity | BMC Genomics | Full Text
https://bmcgenomics.biomedcentral.com/articles/10.1186/1471-2164-14-788
- Porcari S. et al. International consensus statement on microbiome testing in clinical practice. Lancet Gastroenterol Hepatol 10:154‑167 (2025).
- Li F. et al. Highly accurate absolute quantification of bacterial strains in fecal samples. Microbiome 12:168 (2024).
- Ratiner K. et al. Utilization of the microbiome in personalized medicine. Nat Rev Microbiol 22:291‑308 (2024).
- Wilmanski T. et al. Gut microbiome pattern predicts survival in humans. Nat Metab 3:274‑286 (2021).
- Ballard Z. S. et al. Mobile technologies for discovery and engineering of the global microbiome. ACS Nano 12:3065‑3082 (2018).
- Rodriguez J. et al. Future of microbiome‑based biomarkers: Delphi consensus. Lancet Microbe 6:e100948 (2025).