20 August 2025
Reading time [minutes]: 12
Market and Sector Trends
Personalised medicine: potential and investment for the healthcare of the future
Decentralised multiplex molecular diagnostics for faster, scalable and sustainable treatment pathways.
Abstract
Personalised medicine is emerging as a key paradigm for the healthcare of the future, shifting the approach from “one-size-fits-all” therapies to tailored treatments based on the patient's genetic and molecular profile. This article examines the clinical, economic and technological implications of this revolution from a strategic and industrial perspective. We analyse how advances in multiplex molecular diagnostics – decentralised, modular and integrable platforms – enable scalable and sustainable care models, reducing response times and optimising treatment pathways. With academic references and case studies, we will offer insights for biotech investors, clinical boards, OEMs and institutional stakeholders, highlighting the role of Ulisse Biomed as an infrastructure enabler for a new generation of personalised medicine.
Snapshot
-
Personalised (or precision) medicine
Medical approach that adapts prevention, diagnosis and therapy to the patient's individual characteristics (genetic profile, biomarkers, lifestyle), going beyond the standard one-size-fits-all model. Objective: to maximise the effectiveness of treatment and reduce unnecessary or unwanted effects.
-
qPCR multiplex
Quantitative real-time PCR technique capable of detecting multiple genetic targets simultaneously in a single test. It allows the diagnosis of panels of pathogens or biomarkers in parallel (e.g. multiple respiratory viruses in one swab), increasing efficiency and speed compared to single tests.
-
TAT (Turn-Around Time)
Completion time of a diagnostic test, from execution to report. In traditional diagnostics this can be days; point-of-care and multiplex solutions aim to drastically reduce TAT (even to <1 hour) while improving clinical timeliness.
-
Companion diagnostics (diagnostici companion)
Diagnostic tests associated with a specific target drug, performed before or during therapy to determine the presence of a biomarker predictive of response. Example: genetic tests such as EGFR or ALK in oncology that indicate whether a patient will benefit from a certain target drug. Essential for safe and effective personalised therapies.
- Introduction
- 1. Clinical impact: towards tailor-made therapies and better outcomes
- 2. Technological innovation: advanced, decentralised and integrated diagnostics
- 3. Economic impact and sustainable business models
- 4. Innovative revenue models in personalised diagnostics
- 5. Interview with Bruna Munari, Founder and Board Member of Ulisse Biomed
- Projective conclusion
Introduction
Personalised medicine promises to transform healthcare by making it more effective, predictive and patient-centred. Many standard treatments are effective in only a minority of individuals: robust evidence shows that the “one-size-fits-all” model generates clinical and economic inefficiencies (e.g. exposure to ineffective therapies, repeated diagnostic procedures, delayed decision-making) that molecular stratification can prevent from the outset of the treatment pathway [1]. This generic approach leads to wasted resources and additional costs due to adverse effects, repeated diagnostic procedures and delays in finding the right therapy [2]. Personalised medicine was developed to overcome these limitations by combining advances in genomics, data analytics and advanced diagnostics: instead of proceeding by trial and error, patients are stratified into molecular subgroups, targeting the most appropriate treatment for each individual from the outset. This increases the likelihood of a positive response and reduces unnecessary exposure to ineffective drugs [3].
In recent years, several technological convergences have accelerated this vision. The cost of genome sequencing has plummeted (from billions of dollars to a few hundred), while new technologies such as CRISPR and liquid biopsy have opened up unthinkable scenarios for diagnosing and monitoring diseases at the molecular level. At the same time, regulatory bodies and healthcare systems have begun to recognise the value of personalised medicine: in 2018, around 40% of new drugs approved by the FDA were precision therapies (often accompanied by a diagnostic test) [4]. International consortia and government programmes (such as the European ICPerMed and the American PMI) are financially supporting research and implementation, highlighting the global commitment to more efficient and equitable care [5][6]. However, challenges remain: integrating personalised medicine into existing clinical workflows, making it sustainable in terms of costs and infrastructure, and above all scaling these innovations beyond centres of excellence towards widespread adoption.
In this context, molecular diagnostics plays a crucial role as an enabler: rapidly identifying biomarkers and mutations is a prerequisite for timely targeted treatments. Tools such as real-time multiplex PCR, targeted NGS panels and high-sensitivity point-of-care tests form the technical backbone of personalised medicine in the field. Ulisse Biomed, with its Hyris System™ platform and an integrated ecosystem of portable devices, proprietary reagents and cloud services, is positioned precisely in this space – as a technology partner capable of decentralising and modularising advanced molecular diagnostics. Below, we will analyse the clinical, technological and economic potential of this new paradigm, followed by a discussion with Bruna Marini (founder and board member of Ulisse Biomed) on the company's industrial and strategic role.
1. Clinical impact: towards tailor-made therapies and better outcomes
The clinical impact of personalised medicine is already evident in several areas, with oncology leading the way. Targeted therapies tailored to specific mutations (e.g. in EGFR, HER2, BRAF) show significantly higher response rates than standard protocols: in one study, cancer patients treated with drugs selected by biomolecular testing had positive responses in 30–40% of cases, compared with less than 10% of unselected patients [7]. Molecular matching also means greater safety: an analysis of clinical trials found that cancer drugs accompanied by a companion diagnostic had fewer serious adverse events and fewer treatment discontinuations due to toxicity than drugs administered without selection testing [8]. In practice, by identifying in advance which patients may benefit from targeted treatment, others are spared unnecessary side effects, improving the overall tolerability profile.
Another emblematic example is the reduction of unnecessary chemotherapy. Large-scale studies have shown that, by genetically testing breast tumours before deciding on treatment, a significant proportion of patients can avoid chemotherapy: for example, MINDACT indicates that ≈46% of women who are clinically high-risk but low-risk genomically can forego chemotherapy with comparable outcomes. TAILORx shows that for most patients with intermediate scores on the 21-gene test, chemotherapy adds no benefit over endocrine therapy alone [9][10]. Similarly, in metastatic colon cancer, the routine use of genetic testing (e.g., KRAS mutation) to guide treatment choices generates substantial healthcare savings, estimated to be in the hundreds of millions of dollars per year in the US, by avoiding ineffective anti-EGFR therapy in non-responders [11][12]. These data highlight a double advantage: clinical (more appropriate therapies, patients living longer and with a better quality of life) and economic (resources that can be reinvested elsewhere rather than wasted on unsuccessful treatments).
From an operational point of view, personalised medicine makes it necessary to increasingly integrate diagnostics into the treatment pathway. Concepts such as companion diagnostics and theranostics (therapies with built-in diagnostics) are becoming an integral part of protocols: for example, in lung cancer, the diagnostic-therapeutic algorithm now includes immediate molecular tests on biopsy samples to decide whether the patient will receive molecularly targeted therapy or immunotherapy. Personalised tests are also gaining ground outside oncology: pharmacogenetic panels guide drug selection in psychiatry (avoiding ineffective antidepressants based on the patient's metabolism) and genetic scores predict cardiovascular risk and guide preventive measures for high-risk individuals. In summary, the clinical model is evolving: on the one hand, patients receive more targeted and potentially more effective care; on the other hand, clinicians have new decision-making tools based on objective molecular data. International studies confirm that these approaches lead to better overall outcomes and more efficient use of healthcare resources [3].
2. Technological innovation: advanced, decentralised and integrated diagnostics
The revolution in personalised medicine relies heavily on technological advances in molecular diagnostics and data analysis. One of the cornerstones is the ability to perform rapid and accurate multiplex tests, even close to the patient. In the past, identifying multiple markers required complex sequences of tests in a central laboratory; today, syndromic panels and portable devices allow dozens of targets (genes, mutations, pathogens) to be detected simultaneously from a single biological sample [12]. Ulisse Biomed Hyris System™ is an example of a modular platform that integrates miniaturised hardware (bCUBE™ devices for Real-time PCR), proprietary high-stability reagents and cloud software (bAPP™) to manage tests and data remotely [13]. This architecture allows the molecular biology laboratory to be taken wherever it is needed: from the hospital bedside to the peripheral clinic, to non-hospital settings such as pharmacies or emerging countries without large laboratories [13]. Case study: in the field of infectious diseases, the adoption of portable systems in emergency rooms has been shown to reduce diagnostic response times from >24 hours to ~1 hour, with enormous benefits for the immediate initiation of appropriate therapies [12].
The decentralisation of diagnostics made possible by these technologies has a direct impact on clinical models: molecular tests performed on site (Point-of-Care Testing) eliminate delays associated with sample transport and waiting for centralised results [12]. This means that targeted treatment can be started during the same visit or within a few hours instead of days. For example, in patients with suspected respiratory infection, the use of rapid multiplex panels for viruses and bacteria has been shown to increase real-time diagnoses in emergency departments and allow antivirals (such as oseltamivir for influenza) to be administered before discharge, within the optimal window of effectiveness [12]. Studies by the American Society for Microbiology show that multiplex molecular panels have higher sensitivity than traditional methods and provide answers in a timely manner for clinical decisions, with the potential to replace conventional test batteries and improve efficiency and costs [12].
A key aspect of innovation is modularity: modern platforms allow diagnostic capacity to be scaled progressively by adding modules or devices in parallel [13]. This modular and scalable approach is at the heart of Ulisse Biomed's strategy: thanks to hardware-software-reagent verticalisation, each component communicates natively with the others and new modules (both instruments and cloud services) can be integrated seamlessly [13]. For example, an OEM partner may decide to use the Hyris ecosystem by adding its own customised tests, or a hospital may connect external systems via the bGATE™ gateway, integrating all data on a single cloud platform [13]. Interoperability with hospital LIS and external databases is in fact an essential requirement for data-driven personalised medicine [5]; cloud solutions such as bAPP™ allow results from different sources to be centralised and interpretation algorithms (ML/AI) to be applied on a large scale [13].
Finally, the vertical integration of innovative technologies makes it possible to overcome logistical barriers that previously hindered their widespread use in the field. One example is the formulation of ambient-stable reagents that do not require a cold chain: Ulisse Biomed has developed lyophilised kits that can be used at room temperature, eliminating the need for refrigerators for transport and storage [13]. This enables the use of molecular tests in remote or low-resource settings, expanding access to precision medicine. Similarly, sample-to-answer automation (in systems that provide it) and simplified workflows reduce manual steps, allowing even non-specialised personnel to perform genetic tests with ease [12]. In summary, technological innovation is making personalised medicine viable on a large scale, reducing time (real-time results on the cloud), testing costs (through consolidation and multiplexing) and geographical barriers (decentralised portable diagnostics).
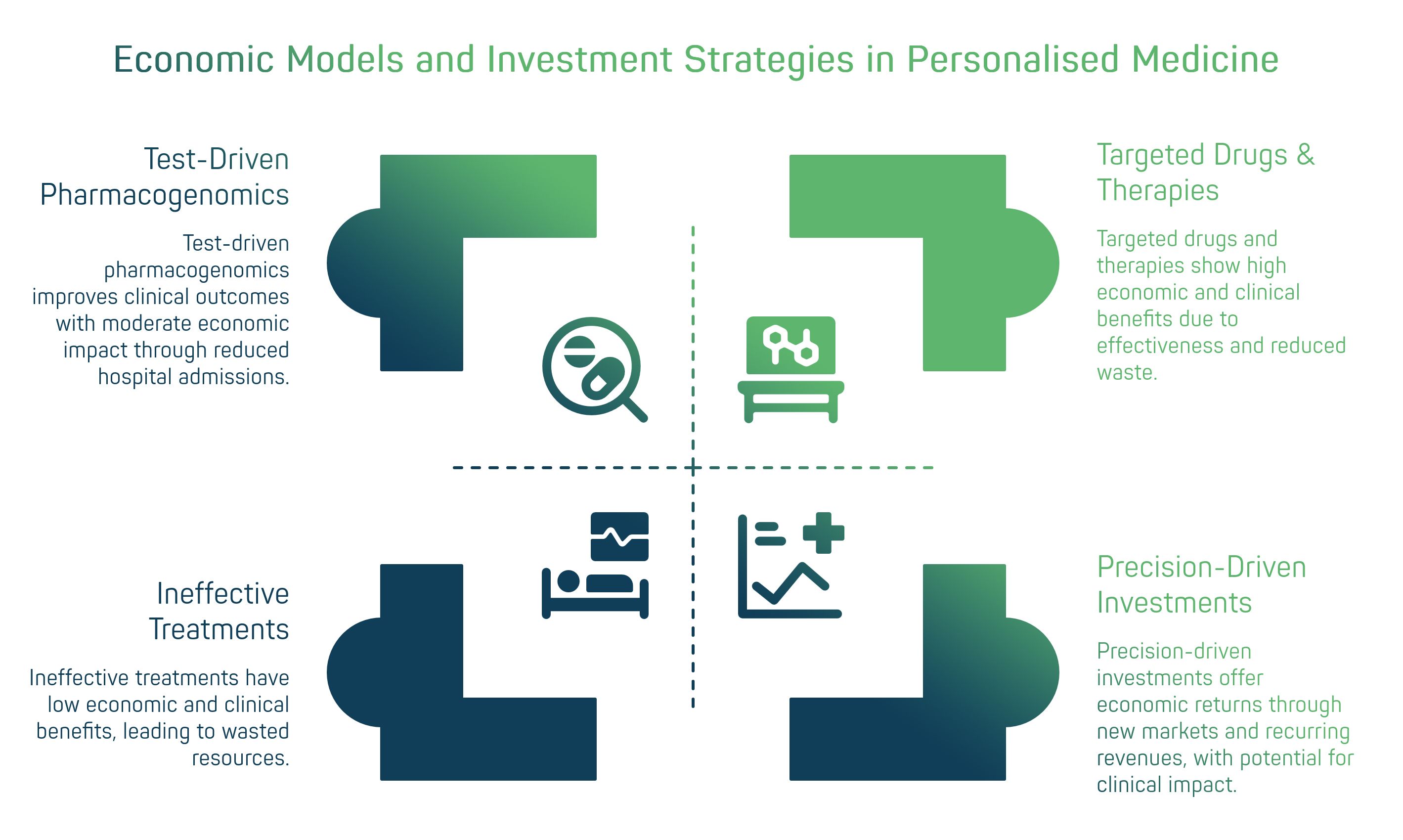
3. Economic impact and sustainable business models
The widespread adoption of personalised medicine inevitably requires a rethinking of economic models in healthcare and the life sciences industry. On the one hand, the costs of developing ultra-targeted therapies and related diagnostic tests can be high; on the other hand, the long-term benefits – both clinical and financial – are promising: more effective therapies mean fewer resources wasted on ineffective treatments and fewer avoidable hospitalisations. Economic evaluations show that value-based care favours personalised interventions when they demonstrate outcomes and cost-effectiveness. At the clinical-organisational level, test-driven pharmacogenomics has been associated with reductions in hospital admissions/emergency room visits compared to usual care, although the magnitude of the effect varies by setting and disease [14][17][18].
From the perspective of the biotech and pharmaceutical industry, personalised medicine is changing investment strategies. Large companies have gradually shifted their focus towards targeted drugs: historical analyses indicate that ~42% of compounds in the pipeline are personalised/biomarker-driven, while in oncology, the importance of “novel” therapeutic modalities (cell & gene, ADC, multispecific) is growing, reflecting the evolution towards targeted approaches [18][15]. Institutional investors are also following the trend: in 2022, VC fundraising in healthcare reached nearly $22 billion (the second-best year on record), and large deals in the “precision-driven” space (e.g., ElevateBio $401 million for gene therapies) demonstrate patient capital's interest in scalable platforms [16][17]. This confidence is motivated by medium-term return opportunities: although initial infrastructure and validation costs are high, the potential ROI includes new markets (gene/cell therapy, data-driven diagnostics), recurring revenues and alignment with outcome-oriented reimbursement policies [14][19].
Market forecasts reflect this expanding scenario, with estimates varying greatly depending on definitions: at the “segment” level, IQVIA forecasts global spending on oncology drugs to reach ~$409 billion by 2028 (the primary driver of precision medicine), while BCC Research estimates the global precision medicine market to be worth ~$100.5 billion in 2028 (CAGR ~13.2%) and companion diagnostics at ~$15.4 billion (CAGR ~15.2%) [15][20][21]. At the same time, new business models are emerging in diagnostics and personalised services: Dx-as-a-Service for facilities that prefer to pay for tests rather than invest in capex, co-development of drugs and diagnostics, and OEM agreements in which platforms such as Hyris System™ are integrated into third-party solutions, with revenues from licensing, reagents and cloud services [13][19].
Ultimately, personalised medicine can only reconcile innovation and sustainability by adopting new industrial approaches. An ecosystem is needed in which public and private stakeholders collaborate: targeted reimbursements and investment programmes on the one hand; on the other, Real-World Evidence to demonstrate economic impact (reduction of waste, complications and hospitalisations). In this context, companies such as Ulisse Biomed can act as infrastructure partners: flexible diagnostic platforms on which hospitals, pharma companies and research centres can build customised verticals [14][13][19].
4. Innovative revenue models in personalised diagnostics
OEM (Original Equipment Manufacturer): Collaboration in which a diagnostics company provides its technology platform for a partner to integrate into its product or service. In the case of Ulisse Biomed, the Hyris System™ platform can be provided on an OEM basis to third-party diagnostic kit developers, who use it as the technical backbone for their customised tests. This model generates revenue through licensing agreements, hardware/reagent supplies and technical support, expanding the reach of Ulisse Biomed's technology in the markets served by its partners.
DaaS (Diagnostics-as-a-Service): A ‘service’ model in which the customer (e.g. a hospital or pharmacy) does not purchase instruments or kits directly, but pays according to usage (per test or on a periodic subscription basis). Ulisse Biomed can offer its diagnostic ecosystem as a service, installing bCUBE devices and providing reagents, cloud services and support, while the customer pays a fee for each analysis performed. This guarantees a recurring revenue stream for the company and lowers the barrier to entry for the end user, who avoids the initial capital-intensive investment.
Co-development: Strategic partnership between a diagnostics company and another entity (e.g., a pharmaceutical biotech, research institute, or healthcare organisation) to jointly develop new tests or customised panels. In such agreements, R&D costs and expertise are shared: Ulisse Biomed can contribute its technology platform and diagnostic validation know-how, while the partner provides samples, access to patients or innovative molecules. Revenue models involve sharing the profits from the commercial success of the new test, as well as possible milestone payments during development. Co-development accelerates innovation (by combining complementary strengths) and creates long-term links with key stakeholders (e.g. by developing a companion diagnostic together with the manufacturer of the related drug).

Bruna Munari
Founder and Board Member of Ulisse Biomed
Interview
Q: Bruna, how would you describe Ulisse Biomed's vision of personalised medicine and its impact on the diagnostics sector? What role does the company aspire to play in this evolving landscape?
A: Our vision is that personalised medicine is not just a scientific trend, but a paradigm shift that requires new diagnostic “infrastructures”. Ulisse Biomed was founded with the aim of providing these infrastructures. In practice, we want to democratise access to advanced molecular diagnostics, taking it out of centralised laboratories and integrating it into everyday care pathways. To do this, we have combined biotech and digital expertise to create a complete ecosystem – the Hyris System™ – where hardware, software and reagents are designed together. In the context of personalised medicine, this means that a hospital, pharmaceutical company or partner can use our platform to perform genetic and molecular tests where and when they are needed, obtaining reliable results in real time and sharing them on the cloud. Essentially, we position ourselves as a technology enabler: we provide the “backbone” on which others can build personalised solutions, from companion tests for cancer therapies to tailor-made panels for a patient's microbiota. We believe that this integrated and distributed approach can have a huge impact: reducing diagnostic times, improving clinical outcomes and making the adoption of precision medicine sustainable on a large scale.
Q: How does multiplex, decentralised and modular molecular diagnostics – such as that offered by the Hyris System™ – enable faster and more scalable clinical models? Can you give us some concrete examples of benefits in the clinical setting?
A: Certainly. Consider, for example, the management of emerging infectious diseases or diagnostics in emergency rooms. Traditionally, to identify a rare pathogen or multiple infectious agents, a sample had to be sent to a reference laboratory, wait perhaps two to three days for the results, and in the meantime treat the patient empirically. With a decentralised, multiplex solution like ours, however, the test is performed directly in the field or in the ward and can simultaneously search for all the main relevant pathogens in a single reaction. The result, available in 90 minutes, for example, allows the doctor to make informed decisions immediately (patient isolation, targeted choice of antibiotic or antiviral). In some pilot hospitals, this has led to a drastic reduction in turnaround time and improved care: patients are treated earlier and more appropriately, with fewer complications and lower hospital costs because patients do not have to be kept in hospital waiting for test results. Another concrete example is that of community medicine or countries with limited resources: thanks to our portable, battery-powered bCUBE devices, healthcare organisations have been able to carry out genetic screening and drug resistance tests directly in remote villages or in the field (I am thinking of a project in Africa to monitor malaria). Decentralising diagnostics means bringing personalised medicine everywhere, not just to large centres: this is crucial to making it truly inclusive. From a technical point of view, having a modular system helps scalability: a partner can start with a few devices and a few tests and then grow by adding other modules as needed – all without changing platforms. This flexibility facilitates adoption, because everyone can tailor the most suitable diagnostic configuration and expand it over time.
Q: Let's look at the business model and investments. Personalised medicine involves significant R&D costs and new ways of collaborating. What revenue models is Ulisse Biomed pursuing to combine technological innovation and economic sustainability?
A: That's a key question. From the outset, we chose to diversify our revenue models precisely to give us flexibility in the emerging market of personalised medicine. First, we adopt a mixed product + service model: we supply diagnostic devices and kits, but integrate them with software and cloud platforms that generate recurring revenue (SaaS or per-test fees). For example, our bAPP cloud offers advanced analytics and continuous AI updates. At the same time, we focus heavily on OEM partnerships and co-development. This means that we don't just sell to the end customer, but also to other industry players: for example, we enter into agreements with pharmaceutical companies to jointly develop a companion diagnostic linked to one of their drugs, sharing both the costs and the benefits of sales. We have OEM agreements where our bCUBE devices are branded or integrated into larger diagnostic partners' solutions, bringing us revenue and amplifying the reach of our technology to markets we would not be able to reach on our own. These collaborative models are win-win: Ulisse Biomed monetises its technology platform without having to be directly present in every niche, and the partner enriches its offering with an innovative layer. From an investment perspective, this diversified approach has also been appreciated by our financiers and shareholders: it reduces risk because we do not depend on a single source of income or a single product success. Finally, we are exploring Diagnostics-as-a-Service formulas, where we install our platforms at large customers and they pay for usage, as an operational service. This model is particularly attractive for clinics and laboratories that want to upgrade to precision diagnostics without having to invest large amounts of capital upfront: we provide the infrastructure, they use it and pay based on volume. In summary, flexibility and partnership are the keywords: in a new sector such as this, we believe that adapting to the needs of various stakeholders is essential to establish ourselves and generate long-term value.
Q: Finally, what challenges and opportunities do you see in the next 5-10 years for Ulisse Biomed and, more generally, for the personalised medicine ecosystem?
A: The next decade will be crucial. In terms of opportunities, we see a huge expansion of application fields: personalised medicine will go beyond oncology to permeate immunology, rare diseases and preventive medicine. There will be a need for diagnostic solutions that are increasingly integrated with clinical data: I am thinking of platforms that combine sequencing, wearables and artificial intelligence to provide doctors with a 360° view of the patient. For Ulisse Biomed, this means continuing to innovate both in the laboratory (new Sagitta panels for emerging biomarkers, more powerful AI algorithms for the analysis of diagnostic big data) and in the field, where we want to consolidate our international presence. One of our strengths will certainly be interoperability: those who manage to connect their systems with hospital digital ecosystems and public databases will have an advantage, because personalised medicine is, by definition, data-driven*. We are working to certify our platform in several countries, integrate it with the main LISs and perhaps leverage global standards such as HL7/FHIR for the exchange of genomic data. As for challenges, I see some important ones. First and foremost, regulation: as tests become more complex (e.g., multigene algorithms, artificial intelligence interpreting diagnostic data), regulators will need to adapt regulations to ensure efficacy and safety without stifling innovation. There will need to be ongoing dialogue between industry and regulators, and companies like ours will need to invest in quality and compliance from the R&D stages onwards. Another challenge is data management and privacy: personalised medicine generates sensitive data (genomes, predispositions) and this must be treated with the utmost care, earning the trust of patients. We are developing cloud architectures with high standards of security and anonymisation because we know that this aspect will also be crucial for social acceptance. Finally, there is a “cultural” challenge: bringing personalised medicine into everyday clinical practice requires training for doctors and laboratory technicians. It is not enough to provide the technology; we must explain and demonstrate its added value.
In conclusion, I am very confident: I believe that Ulisse Biomed has the advantage of having been founded with this mission and innovative DNA. We are agile and focused. If we continue to put patients and outcomes at the centre, working alongside clinicians and researchers, I am convinced that we will not only seize opportunities but also actively contribute to shaping a truly effective and sustainable personalised medicine ecosystem.
Projective conclusion
Personalised medicine represents a sea change for healthcare systems and the life sciences industry. From a strategic perspective, its clinical potential (better outcomes, tailored therapies, proactive prevention) goes hand in hand with unprecedented technological and economic opportunities. As we have seen, innovative diagnostic tools – from portable multiplex panels to cloud-based analysis of genomic big data – are transforming the way we collect and use patient information, bringing diagnosis closer to the point of care and reducing traditional bottlenecks. At the same time, investors and institutional stakeholders are increasingly recognising the value of these models: substantial capital is flowing into the sector and emerging healthcare policies aim to support approaches that offer greater efficiency and quality than the status quo.
Challenges certainly remain, from the financial sustainability of high-cost therapies to the need for solid evidence of real-world benefits. But the medium-term outlook appears favourable: the vision for 2030 outlined by international consortia is that of a healthcare system in which personalised medicine is fully integrated, with a tangible impact in terms of public health and economic development[5][6]. We can look forward to a future in which every patient will have access to rapid, targeted molecular tests, with results that can be used immediately to choose the optimal therapy; a future in which the data of millions of people, appropriately aggregated and analysed, will guide new discoveries and more effective prevention policies; a future in which collaborative business models will bring industry, the public system and research together towards the common goal of more effective, safer and more sustainable care for the population.
Ulisse Biomed intends to play a leading role in this future. As highlighted, the company positions itself as an infrastructure enabler, providing technologies and solutions that enable the practical implementation of personalised medicine on an industrial scale. From the Hyris System™ platform, which decentralises diagnostics while maintaining high standards, to its ability to create co-development partnerships, Ulisse Biomed builds bridges between innovation and practical application. The path is clear: investing in personalised medicine means investing in smarter, data-driven healthcare that combines scientific rigour with strategic vision. The clinical and technological potential is there, and investment is coming: the challenge for the coming years will be to turn these promises into everyday reality, making personalised medicine not a privilege for the few but a standard for all. With its solid clinical, technical and financial foundations, Ulisse Biomed aims to lead this change as a partner of choice for institutions and companies in building the healthcare system of the future.
Sources and Bibliography
- Cleary, M. A Review of Precision Medicine, Companion Diagnostics, and the Challenges Surrounding Targeted Therapy. Value & Outcomes Spotlight (ISPOR). July/August 2019. PDF
- Ocaña, A. et al. Influence of companion diagnostics on efficacy and safety of targeted anti-cancer drugs: systematic review and meta-analyses. Oncotarget. 2015;6(37):39538–39549. PMCID: PMC4741844 · DOI
- Vicente, A.M. et al. How personalised medicine will transform healthcare by 2030: the ICPerMed vision. Journal of Translational Medicine. 2020;18:180. Full text · DOI
- Personalized Medicine Coalition (PMC). Personalized Medicine at FDA: A Progress & Outlook Report (2018). 2019. PDF
- ICPerMed. The ICPerMed Vision for 2030: How can personalised approaches pave the way to next-generation medicine? 2019. PDF
- White House (archived). FACT SHEET: President Obama’s Precision Medicine Initiative. Jan 30, 2015. Link
- Schwaederle, M. et al. Association of biomarker‑based treatment strategies with response rates and progression‑free survival in refractory malignant neoplasms: a meta‑analysis. JAMA Oncology. 2016;2(11):1452–1459. Article · DOI
- Cardoso, F. et al. 70‑Gene Signature as an Aid to Treatment Decisions in Early‑Stage Breast Cancer (MINDACT). New England Journal of Medicine. 2016;375:717–729. Article · DOI
- Sparano, J.A. et al. Adjuvant Chemotherapy Guided by a 21‑Gene Expression Assay in Breast Cancer (TAILORx). New England Journal of Medicine. 2018;379:111–121. Article · DOI
- Behl, A.S. et al. Cost‑Effectiveness Analysis of Screening for KRAS and BRAF Mutations in Metastatic Colorectal Cancer. Journal of the National Cancer Institute. 2012;104(23):1785–1795. PMCID: PMC3514165 · DOI
- Kircher, S.M.; Mohindra, N.; Nimeiri, H. Cost Estimates and Economic Implications of Expanded RAS Testing in Metastatic Colorectal Cancer. The Oncologist. 2015;20(1):14–18. PMCID: PMC4294607 · DOI
- American Society for Microbiology (ASM). Clinical Utility of Multiplex Tests for Respiratory and GI Pathogens. Guideline, Aug 23, 2019. Webpage
- Ulisse Biomed. The Hyris System™ modular diagnostic platform (ecosystem overview, bCUBE™, bAPP™, bGATE™, reagenti liofilizzati). ID15 (EN) · bAPP · bGATE · (IT, reagenti liofilizzati)
- Chen, W. et al. Evaluating the Value for Money of Precision Medicine from Early Cycle to Market Access: A Comprehensive Review of Approaches and Challenges. Value in Health. 2023;26(9):1425–1434. Full text · DOI
- IQVIA Institute. Global Oncology Trends 2024: Outlook to 2028. Report
- Silicon Valley Bank (SVB). Healthcare Investments & Exits — Annual Report 2022. Report page · Slides (NVCA/SVB)
- Axios. ElevateBio raises $401 million in year’s largest biotech VC deal. 2023. Article · Press release
- Personalized Medicine Coalition (PMC). The Personalized Medicine Report — Opportunity, Challenges, and the Future (dataset update through 2020; includes pipeline analysis). PDF · Alt. PDF (pipeline 42%)
- Koleva‑Kolarova, R. et al. Financing and Reimbursement Models for Personalised Medicine: A Systematic Review to Identify Current Models and Future Options. Applied Health Economics and Health Policy. 2022;20(4):501–524. PMCID: PMC9206925 · DOI
- BCC Research. Precision Medicine: Global Markets (2019–2028). Report page
- BCC Research. Companion Diagnostics: Technologies & Global Markets (2023–2028). Report page · Press note